Community Annotated Trial Search (CATS)
Digital platform for efficient management and filtering
of clinical trials through semantic annotations
Optimization of clinicaltrial management
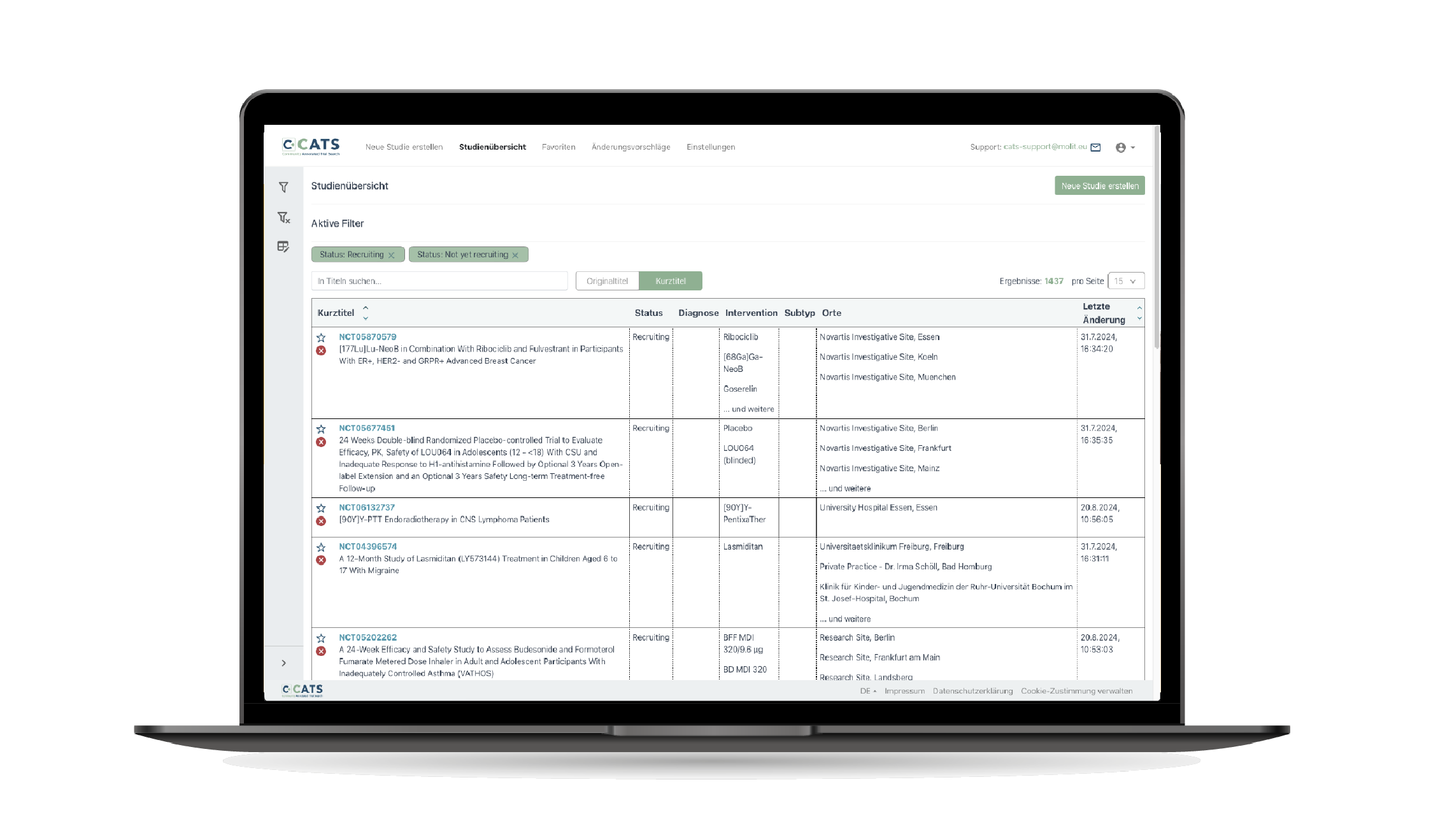
CATS is a digital, interoperable platform for managing trial information. It integrates semi-automatically with established study information systems (such as clinicaltrials.gov, German Register of Clinical Trials, …) and supplements these with semantic annotations. This enables precise filtering according to clinical characteristics and local conditions. The modern data model supports complex oncological trial designs and allows filtering by exclusion and inclusion criteria.
CATS is based on an interoperable data and information model defined in HL7 FHIR. All definitions are publicly and freely available in an implementation guide, which should simplify adoption in the community and the use of the interfaces.

An HL7-based Clinical Decision Support Hook (CDS Hook) was developed for the Molecular Tumor Board (MTB), which automatically suggests patient-specific studies. Scoring algorithms based on the DMN™ standard of the Object Management Group are used to evaluate the studies. Thanks to the high degree of standardization, the CDS hook could be seamlessly integrated into the MOLIT framework, including the MTB software VITU.
MOLIT CATS Team

Dr. Stefan Sigle
Product owner
Principal Investigator

Georg Mathes
Full-Stack Developer

Felix Edel
CDS Hook development, Backend

Nathalie Block
Design, UX

Chantal Bachschmid
Community Management

Patrick Werner
(Former) Interoperability advisor
Functions of the study platform

Automated Annotation
CATS automatically supplements study information with semantic annotations to enable more precise and efficient filtering for relevant studies.

Community Data Input
CATS allows the community to contribute information such as local proximity searches, improving the timeliness of studies.

Flexible Data model
CATS' flexible data model allows complex oncology studies with multiple cohorts to be managed and filtered in detail by exclusion and inclusion criteria, which is particularly beneficial in personalized medicine.
Timeline
Current milestones and results
March 2021
First Draft of the
CATS platform
completed
June 2021
Release of the automated
study annotation
August 2021
Release Clinical
Decision Support Hooks
April 2022
Internal Release
March 2023
Release UI redesign
July 2024
Connection of the first
external Partners (UK HD) via API
August 2024
First Public Release
Contact
For further information or
if you have any questions, please contact

Dr. Stefan Sigle
Head of Data Science & AI
Mail Stefan.Sigle@molit.eu
Phone 07131 / 13345-42